Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:3 Percentile:68.71(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U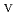
 Fe
Fe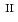
 U
U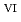 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
Yamada, Ryohei; Odagiri, Taiki*; Iwaoka, Kazuki*; Hosoda, Masahiro*; Tokonami, Shinji*
Radiation Environment and Medicine, 8(1), p.21 - 25, 2019/02
We evaluate radon/thoron and its progeny concentration using passive-type monitors using CR-39 plates. After exposure, it is necessary to do chemical etching for CR-39 plates. In the present study, we considered shortening of chemical etching time for CR-39 and enlargement of the track diameter (i.e. etch pit diameter) aiming for introduction of automatic counting system in the future. Optimum conditions were determined by changing solution concentration, solution temperature and etching time. As a result, the optimized conditions (concentration, temperature and etching time) were determined to be 8 M NaOH solution, 75 degrees Celsius and 10 hours. This result of etching time showed that the chemical etching was completed in less than half of conventional etching time. Furthermore, it was suggested that shorter etching time would be possible if we do not consider the enlargement of conventional track diameter.
Mukai, Yasunobu; Nakamichi, Hideo; Kobayashi, Daisuke; Nishimura, Kazuaki; Fujisaku, Sakae; Tanaka, Hideki; Isomae, Hidemi; Nakamura, Hironobu; Kurita, Tsutomu; Iida, Masayoshi*; et al.
Proceedings of 2017 International Congress on Advances in Nuclear Power Plants (ICAPP 2017) (CD-ROM), 8 Pages, 2017/04
TRP has stored the plutonium in solution state for long-term since the last PCDF operation in 2007 was finished. After the great east Japan earthquake in 2011, JAEA had investigated the risk against potential hazard of these solutions which might lead to make hydrogen explosion and/or boiling of the solution accidents with the release of radioactive materials to the public when blackout. To reduce the risk for storing Pu solution (about 640 kg Pu), JAEA planned to perform the process operation for the solidification and stabilization of the solution by converted into MOX powder at PCDF in 2013. In order to perform PCDF operation without adaption of new safety regulation, JAEA conducted several safety measures such as emergency safety countermeasures, necessary security and safeguards (3S) measures with understanding of NRA. As a result, the PCDF operation had stared on 28th April, 2014, and successfully completed to convert MOX powder on 3rd August, 2016 for about 2 years as planned.
Tsuru, Tomohito; Chrzan, D. C.*
Scientific Reports (Internet), 5, p.8793_1 - 8793_8, 2015/03
Times Cited Count:68 Percentile:86.71(Multidisciplinary Sciences)Much of the world's future economy hedges on the ability to improve the efficiency of mechanical machines without sacrificing their performance. This requires the development of lighter and stronger structural alloys. Magnesium alloys are very promising for structural applications, but they suffer from a fatal flaw. The mobility of the dislocations is determined by the bonding within the alloy, and this bonding is best modeled using approaches rooted firmly in quantum mechanics. Here, we compute the electronic structure of a screw dislocation gliding on a prismatic plane within Mg as it passes over its Peierls barrier. We show that the ductility of Mg is increased because some substitutional additions strongly bind to and stabilize a compact core structure for screw dislocations that are formally able to glide on both the basal and prismatic planes.
Haraga, Tomoko; Kameo, Yutaka; Nakashima, Mikio
Bunseki Kagaku, 55(1), p.51 - 54, 2006/01
Times Cited Count:4 Percentile:14.45(Chemistry, Analytical)A relatively large quantity of sample solutions have to be prepared for radiochemical analysis of solidified products yielded by plasma melting treatment of non-metallic radioactive wastes. In order to dissolve the solidified products sample rapidly, dissolution method with microwave heating devices was applied. In a conventional method only by external heating with various mixtures of acids (HNO , HF, HClO
, HF, HClO and H
and H SO
SO ), a 0.1 g amount of the sample was dissolved with difficulty. However, applying the microwave assisted dissolution method, a 1 g amount of the sample was completely dissolved in a shorter time. Thereby the time for dissolution procedures was shortened less than a one-tenth. The present dissolution method was successfully applied to the blast furnace slag as a reference material to determine main elements with good precision.
), a 0.1 g amount of the sample was dissolved with difficulty. However, applying the microwave assisted dissolution method, a 1 g amount of the sample was completely dissolved in a shorter time. Thereby the time for dissolution procedures was shortened less than a one-tenth. The present dissolution method was successfully applied to the blast furnace slag as a reference material to determine main elements with good precision.
Arai, Yasuo; Minato, Kazuo
Journal of Nuclear Materials, 344(1-3), p.180 - 185, 2005/09
Times Cited Count:24 Percentile:82.03(Materials Science, Multidisciplinary)no abstracts in English
Tonoike, Kotaro; Miyoshi, Yoshinori; Okubo, Kiyoshi
Journal of Nuclear Science and Technology, 40(4), p.238 - 245, 2003/04
Times Cited Count:2 Percentile:19(Nuclear Science & Technology)The reactivity effect of neutron interaction between two identical units containing low enriched (10%  enrichment) uranyl nitrate solution was measured in the STACY. The unit has 350mm of thickness and 690mm of width and distance between those two units was adjustable from 0mm to 1450mm. Condition of the solution was about 290gU/L in uranium concentration, about 0.8N in free nitric acid molarity, 24
enrichment) uranyl nitrate solution was measured in the STACY. The unit has 350mm of thickness and 690mm of width and distance between those two units was adjustable from 0mm to 1450mm. Condition of the solution was about 290gU/L in uranium concentration, about 0.8N in free nitric acid molarity, 24 27
27 C in temperature and about 1.4g/cm
C in temperature and about 1.4g/cm in solution density. The reactivity effect was estimated from variation of critical solution level from 495mm to 763mm depending on the core distance. The reactivity effect was also evaluated by the solid angle method and a computational method using the continuous energy Monte Carlo code MCNP-4C and the nuclear data library JENDL3.2. Comparison of those estimations is presented.
in solution density. The reactivity effect was estimated from variation of critical solution level from 495mm to 763mm depending on the core distance. The reactivity effect was also evaluated by the solid angle method and a computational method using the continuous energy Monte Carlo code MCNP-4C and the nuclear data library JENDL3.2. Comparison of those estimations is presented.
Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Minato, Kazuo; Numata, Masami
Proceedings of GLOBAL2003 Atoms for Prosperity; Updating Eisenhower's Global Vision for Nuclear Energy (CD-ROM), p.2285 - 2291, 2003/00
Stability of AmN and (Am,Zr)N was studied comparatively from the viewpoints of the hydrolytic and evaporative behavior. AmN powder reacted with moisture to form hydroxide Am(OH) , while the solid solution (Am
, while the solid solution (Am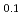 Zr
Zr )N remained stable as long as 1000 hours. Stabilization effect of ZrN was found to depend significantly on its mole fraction from the experiments on (Dy,Zr)N. In the oxidation experiments on (Dy,Zr)N by TG-DTA technique, rapid weight gain by the oxidation occurred above 700 K. Effect of ZrN on the stability against oxygen was slight. Nitrogen release by the evaporation of AmN and (Am
)N remained stable as long as 1000 hours. Stabilization effect of ZrN was found to depend significantly on its mole fraction from the experiments on (Dy,Zr)N. In the oxidation experiments on (Dy,Zr)N by TG-DTA technique, rapid weight gain by the oxidation occurred above 700 K. Effect of ZrN on the stability against oxygen was slight. Nitrogen release by the evaporation of AmN and (Am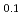 Zr
Zr )N in He gas flow was measured by gas chromatography. Evaporation rate constants of AmN were obtained at 1623-1733 K. Although the evaporation rate constant of AmN in the solid solution were lower than those of the pure AmN, the selective evaporation of AmN from the solid slution were recognized, which resulted in a decrease in the Am mole fraction.
)N in He gas flow was measured by gas chromatography. Evaporation rate constants of AmN were obtained at 1623-1733 K. Although the evaporation rate constant of AmN in the solid solution were lower than those of the pure AmN, the selective evaporation of AmN from the solid slution were recognized, which resulted in a decrease in the Am mole fraction.
Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Ogawa, Toru
Journal of Alloys and Compounds, 327(1-2), p.235 - 239, 2001/08
Times Cited Count:13 Percentile:60.77(Chemistry, Physical)no abstracts in English
 U
U O
O
Fujino, Takeo*; Sato, Nobuaki*; Yamada, Kota*; *; Fukuda, Kosaku; Serizawa, Hiroyuki; Shiratori, Tetsuo
Journal of Nuclear Materials, 265(1-2), p.154 - 160, 1999/00
Times Cited Count:6 Percentile:45.57(Materials Science, Multidisciplinary)no abstracts in English
Fujino, Takeo*; *; Sato, Nobuaki*; Yamada, Kota*; Fukuda, Kosaku; Serizawa, Hiroyuki; Shiratori, Tetsuo
Journal of Nuclear Materials, 246(2-3), p.150 - 157, 1997/00
Times Cited Count:22 Percentile:83.3(Materials Science, Multidisciplinary)no abstracts in English
 solid solution
solid solutionSerizawa, Hiroyuki; Shiratori, Tetsuo; Fukuda, Kosaku; Fujino, Takeo*; Sato, Nobuaki*
Journal of Alloys and Compounds, 218, p.149 - 156, 1995/00
Times Cited Count:3 Percentile:37.09(Chemistry, Physical)no abstracts in English
 -UZr
-UZr at elevated temperatures
at elevated temperaturesAkabori, Mitsuo; Ogawa, Toru; Ito, Akinori; Morii, Yukio
Journal of Physics; Condensed Matter, 7, p.8249 - 8257, 1995/00
Times Cited Count:46 Percentile:89.28(Physics, Condensed Matter)no abstracts in English
 and
and  (
( ) rays from solid and solution sources with several solid scintillators
) rays from solid and solution sources with several solid scintillatorsUsuda, Shigekazu; Mihara, Akira; Abe, Hitoshi
Nuclear Instruments and Methods in Physics Research A, 321, p.247 - 253, 1992/00
Times Cited Count:21 Percentile:84.73(Instruments & Instrumentation)no abstracts in English
; Arai, Yasuo; Iwai, Takashi; Omichi, Toshihiko
Journal of Nuclear Science and Technology, 28(7), p.689 - 691, 1991/07
no abstracts in English



 U
U


 O
O

 solid solution
solid solutionUgajin, Mitsuhiro; ; Shiba, Koreyuki
Journal of Nuclear Materials, 116(2-3), p.172 - 177, 1983/00
Times Cited Count:15 Percentile:81.67(Materials Science, Multidisciplinary)no abstracts in English


 solid solutions
solid solutionsUgajin, Mitsuhiro
Journal of Nuclear Materials, 110, p.140 - 146, 1982/00
Times Cited Count:24 Percentile:88.76(Materials Science, Multidisciplinary)no abstracts in English
 -NdO
-NdO

 and PuO
and PuO -YO
-YO

 solid solutions
solid solutions; ; ;
Journal of Nuclear Science and Technology, 19(8), p.681 - 683, 1982/00
Times Cited Count:2 Percentile:43.95(Nuclear Science & Technology)no abstracts in English

; ; ; *
Journal of Nuclear Science and Technology, 17(1), p.83 - 85, 1980/00
Times Cited Count:0 Percentile:0.29(Nuclear Science & Technology)no abstracts in English
; *
Journal of the Physical Society of Japan, 31(4), p.1056 - 1068, 1971/10
Times Cited Count:81no abstracts in English